Thermophoresis in self-associating systems: probing poloxamer micellization by opto-thermal excitation
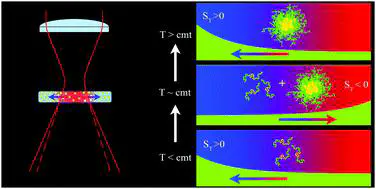 Image credit: RSC
Image credit: RSCAbstract
Due to its exquisite sensitivity to interfacial properties, thermophoresis, i.e., particle motion driven by thermal gradients, can provide novel, exclusive, and often surprising information on the structural properties of colloidal or macromolecular fluids and on particle/solvent interactions at the nanoscale. Here, by using an all-optical thermal excitation technique, thermal lensing, we show that thermophoresis can be profitably exploited to investigate the self-association of an amphiphilic block copolymer, poloxamer P407, which takes place above a concentration-dependent critical micellization temperature (cmt). In particular we show that, around and above the cmt, the direction of the poloxamer thermophoretic motion displays a remarkable double sign inversion, which is fully correlated with a peak in the thermal expansivity of the solution marking the progressive dehydration of the propylene oxide groups of P407 and their incorporation into the micellar core. This rather puzzling behaviour of the thermophoretic mobility and of the Soret coefficient in the P407 micellization region can tentatively be explained by properly taking into account the temperature-dependent balance between micellized and nonassociated poloxamer chains.